What is the maximum number of electrons that can occupy the second energy level (n=2)?
Options:A) 2 electrons
B) 18 electrons
C) 8 electrons
D) 32 electrons
Show Answer
The correct answer is C .
Zn + 2HCL ZnCl
What happens to zinc in the above reaction?
Options:A) oxidized
B) a reactant
C) reduced
D) a metal
Show Answer
The correct answer is A .
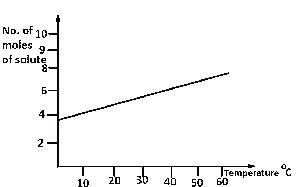
From the diagram above, find the amount of solute deposited when 200 cm3 of the solution is cooled from 55oC to 40oC
Options:A) 0.10 mole
B) 0.20mole
C) 0.01 mole
D) 0.02 mole
Show Answer
The correct answer is B .
A) diffusion
B) migration
C) reaction
D) sedimentaion
Show Answer
The correct answer is B .
A) +7 to +3
B) +6 to +3
C) +5 to +3
D) -2 to +3
Show Answer
The correct answer is A .
A) perspex
B) bakeelite
C) polystyrene
D) polyacrylonitrile
Show Answer
The correct answer is A .
A) is a transition element
B) is at the bottom of the activity series
C) is very reactive
D) has completely filled 3d-orbitals
Show Answer
The correct answer is B .
A chemical process in which there is a gain of electrons is known as?
Options:A) Sublimation
B) Reduction
C) Oxidation
D) Distillation
Show Answer
The correct answer is B .
The dehydration of CH
A) HC≡CCH
B) CH
C) CH
D) CH
Show Answer
The correct answer is D .
A) Mass spectrometric experiment
B) Oil-drop experiment
C) Scattering α - particles
D) Discharge-tube experiment
Show Answer
The correct answer is B .