A) Copper
B) Iron
C) Lead
D) Potassium
E) Zinc
Show Answer
The correct answer is D .
On which of the following is the solubility of a gaseous substance dependent?
I. Nature of solvent
II. Nature of solute
III. Temperature
IV. Pressure
Options:A) I, II, III and IV
B) I and II only
C) II only
D) I, III and IV only
Show Answer
The correct answer is D .
A) CH2O
B) C3H6O3
C) C6H6O3
D) C6H12O6
Show Answer
The correct answer is D .
Which of the following best represent solid gas mixture?
Options:A) milk
B) kerosene
C) soil
D) smoke
Show Answer
The correct answer is D .
In the reaction above, an increase in pressure will Options:
A) decelerate the reaction
B) Increase the yield of
C) increase the yield of
D) accelerate the reaction
Show Answer
The correct answer is C .
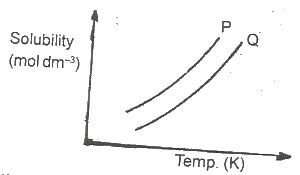
In the diagram above, the mixture of the solids P and Q can be separated by
Options:A) distillation
B) fractional distillation
C) crystallization
D) fractional crystallization
Show Answer
The correct answer is C .
A) deplete oxygen which is necessary for the survival of aquatic organism
B) increase oxygen which is necessary for survival of aquatic organism
C) increase other gaseous substances which are necessary for the survival of aquatic organisms
D) deplete other gaseous species which is necessary for the survival of aquatic organisms
Show Answer
The correct answer is A .
What happens when alkanoic acids react with alcohols in the presence of an acid catalyst?
Options:A) Saponification
B) Esterification
C) Polymerization
D) Hydrolysis
Show Answer
The correct answer is B .
A) 2SO3(g) ↔ 2SO2(g) + O2(g)
B) 2CO2(g) ↔ 2CO(g) + O2(g)
C) 2H2(g) + O2(g) ↔ 2H2O(g)
D) 2NO(g) ↔ N2(g) + O2(g)
Show Answer
The correct answer is C .
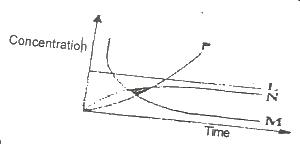
2HCI(ag) + CaCO3(s) → CaCl2(s) + CO 2(g) +H2O(1)
From the reaction above, which of the curves in the diagram represents the production of carbon (IV) oxide as dilute HCI is added?
Options:A) L
B) M
C) N
D) P
Show Answer
The correct answer is B .