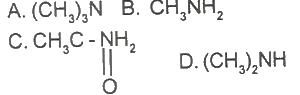
Choose the correct answer in the option above.
Which of the following is a primary amine?
Options:A) A
B) B
C) C
D) D
Show Answer
The correct answer is B .
A) there are no electrons around the nucleus
B) the number of protons equals the number of electrons
C) there are equal number of protons and neutrons in the nucleus
D) the outer electron shell is completely filled
E) the atoms contain only protons
Show Answer
The correct answer is D .
A) - 2792
B) + 2792
C) - 64
D) + 64
Show Answer
The correct answer is D .
A) oxidize it
B) decompose it
C) minimize its decomposition
D) reduce it to water and oxygen
Show Answer
The correct answer is C .
Which halogen is a gas at room temperature and is pale yellow in color?
Options:A) Chlorine (Cl)
B) Fluorine (F)
C) Iodine (I)
D) Bromine (Br)
Show Answer
The correct answer is B .
A) sulphur (IV) oxide
B) carbon (IV) oxide
C) nitrogen (II) oxide
D) hydrogen sulphide
Show Answer
The correct answer is D .
What are the possible oxidation numbers of an element if its atomic number is 17?
Options:A) -1 and 7
B) -1 and 6
C) -3 and 5
D) -2 and 6
Show Answer
The correct answer is A .
2H2(g) + O2(g) → 2H2 O(g)
In the reaction above, what volume of hydrogen would be left over when 300 cm3 of oxygen and 1000 cm3 of hydrogen are exploded in a sealed tube?
Options:A) 200 cm3
B) 400 cm3
C) 600 cm3
D) 700 cm3
Show Answer
The correct answer is B .
In troposphere, the two types of pollutants are?
Options:A) Gaseous and particulate pollutants
B) Carbon and metallic pollutants
C) Natural and man-made pollutants
D) Sulphuric and nitrogenous pollutants
Show Answer
The correct answer is A .
An organic compound which liberate carbon(iv)oxide from trioxocarbonate(iv) solution is likely to be?
Options:A) C
B) C
C) C
D) CH
Show Answer
The correct answer is D .