A) nitrogen
B) hydrogen
C) oxygen
D) flourine
Show Answer
In the reaction between sodium hydroxide and tetraoxosulphate (VI) solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralize 10cm
A) 25cm
B) 10cm
C) 20cm
D) 50cm
Show Answer
rn(C=12,H =1, O=16) Options:
A) C 5H 12O
B) C 3H 8O
C) C 6H 13O 2
D) C 4H 9O
Show Answer
Chlorine, consisting of two isotopes of mass numbers 35 and 37, has an atomic mass of 35.5.
The relative abundance of the isotope of mass number 37 is?
Options:A) 20
B) 25
C) 50
D) 75
Show Answer
In order to electroplate spoon with silver, the arrangement of the electrolytic cell is?
Options:A) the anode is a silver rod and the cathode is the spoon
B) the anode is the spoon and the cathode is a silver rod
C) the electrolyte is silver trioxonitrate(v)( solution and the cathode is a silver rod.
D) the electrolyte is silver trioxonitrate(v) solution and the anode is the spoon
Show Answer
Duralumin consists of aluminum, copper?
Options:A) Zinc and Gold
B) Lead and Manganese
C) Nickel and Silver
D) Manganese and Magnesium
Show Answer
Which of the following reactions is an oxidation process?
Options:A) 2H
B) 2O
C) OCl
D) Cl + e
Show Answer
A) Granulated sugar
B) Sea-water
C) Sodium chloride
D) Iron fillings
Show Answer
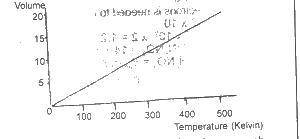
Which of the gas laws does this graph illustrate?
Options:A) Boyle
B) Charles
C) Gay-Lussac
D) Graham
Show Answer
Which of the following mixtures is an example of a colloid?
Options:A) Milk
B) Orange juice
C) Saltwater
D) Sugar dissolved in water
Show Answer