A) The bubbling of chlorine into water
B) The bubbling of chlorine into a jar contanining hydrogen
C) The dissolution of sodium chloride in water
D) The passing of steam over heated iron
Show Answer
The correct answer is C .
The molecular shape and bond angle of water are respectively
Options:A) linear, 180°
B) bent, 109.5°
C) tetrahedral, 109.5°
D) bent, 105°
Show Answer
The correct answer is D .
A) 78 protons and 55 elections
B) 55 protons and 78 neutrons
C) 55 neutrons 78 electrons
D) 78 protons and 55 neutrons
Show Answer
The correct answer is B .
A) Zin heated to redness reacts with steam to give oxygen and zinc oxide
B) Zinc heated to redness reacts with steam to give oxygen and hydrogen
C) Zinc does not react with hot or cold water
D) Zinc reacts with hot water to form zinc oxide and hydrogen
E) Zinc reacts very easily with cold water to give zinc oxide and hydrogen
Show Answer
The correct answer is D .
A) NO
B) CO
C) SO2
D) CuO
Show Answer
The correct answer is C .
H
The equation above illustrates
Options:A) precipitation
B) hydration
C) hydrolysis
D) neutralization
Show Answer
The correct answer is D .
A) P will form an electrovalent bond with R
B) Q will form a covalent bond with S
C) R will form an electrovalent bond with S
D) Q will form an electrovalent bond with S
E) Q will form a covalent bond with R
Show Answer
The correct answer is D .
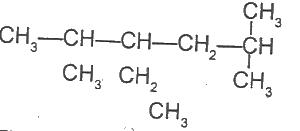
The IUPAC nomenclature of the organic compound with the above structural formula is
Options:A) 3-ethyl-2,5-dimethylhexane
B) 4-ethyl-2,5-dimethylhexane
C) 3-ethyl-2,5,5 - trimethylpentane
D) 3-ethyl, 1,1,4-trimethylpentane
Show Answer
The correct answer is A .
What technique is suitable for separating a binary solution of potassium chloride and potassium trioxochlorate (V)?
Options:A) Fractional crystallization
B) Fractional distillation
C) Filtration
D) Evaporation
Show Answer
The correct answer is A .
A) Volume
B) Mass
C) Pressure
D) Temperature
Show Answer
The correct answer is D .