A) Carbonyl group
B) Hydroxyl groupd
C) Phenyl group
D) Methyl group
Show Answer
The correct answer is B .
A) dissolution
B) slaking
C) liming
D) mortaring
Show Answer
The correct answer is B .
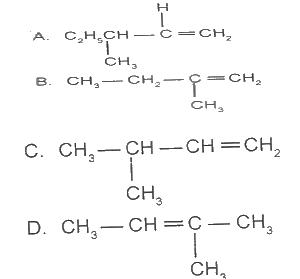
2-methylbut-2-ene has the structure
Options:A) A
B) B
C) C
D) D
Show Answer
The correct answer is D .
C(s) + 2S(g) → CS2(g)
ΔH = +89 kJ mol-1
The chemical equation above implies that
Options:A) each of carbon and sulphur has 89 kJ of energy
B) both carbon and sulphur contribute 89 kJ of energy
C) 89 kJ of energy is released
D) 89 kJ of energy is absorbed
Show Answer
The correct answer is D .
A) cathode ray tube
B) glass tube and discharge tube
C) discharge tube with terminal cathode
D) discharge tube with central cathode
Show Answer
The correct answer is D .
Which of the following is a set of neutral oxides?
Options:A) N
B) N
C) N
D) N
Show Answer
The correct answer is A .
The only metal that is anti-bacterial is?
Options:A) Iron
B) Sodium
C) Alluminium
D) Copper
Show Answer
The correct answer is D .
A) Freezing ice cream
B) dissolving calcium in water
C) burning kerosene
D) Exposing white phosphorus to air
Show Answer
The correct answer is A .
A) 15.00cm3
B) 6.30cm3
C) 0.63cm3
D) 0.06cm3
Show Answer
The correct answer is B .
A) Bomb calorimeter
B) Catalytic cracker
C) Fractionating column
D) Thermometer
Show Answer
The correct answer is C .