A) ethyne
B) diethyl sulphate
C) diethyl ether
D) acetone
Show Answer
The correct answer is C .
What volume of 0.100M sodium trioxonitrate (V) solution contains 5g of solute.
[Na = 23, N = 14, O = 16]
Options:A) 1.7 litres
B) 0.420 litres
C) 0.588 litres
D) 2.35 litres
Show Answer
The correct answer is C .
L . Evaporation. ll. Sublimation. lll. Diffusion. IV. Brownian motion.
Which of the above can correctly be listed as evidences for the particulate nature of matter?
Options:A) I and lll only
B) ll and lV only
C) l, ll and lll only
D) l, ll, lll and lV
Show Answer
The correct answer is D .
A) sodium trioxonitrate (V) and calcium chloride
B) sodium dioxonitrate (III) and ammonium chloride
C) sodium trioxonitrate (V) and ammonium chloride
D) sodium dioxonitrate (III) and potassium chloride
Show Answer
The correct answer is A .
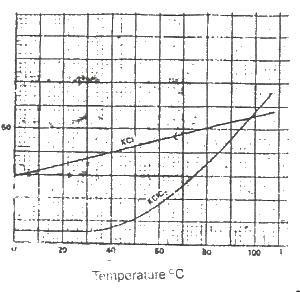
In the solubility curve above, water at 98oC is saturated with KCL and KCIO3 What is the percentage of KCL impurity in the crystals formed when the solution is cooled to 30 oC?
Options:A) 51.5
B) 45.5
C) 34.5
D) 26.5
Show Answer
The correct answer is C .
A) Dilute ammonia
B) Benedict's solution
C) Tollen's reagent
D) Sodium hydroxide solution
Show Answer
The correct answer is C .
A) Calcium reacts with water to form calcium
B) Magnesium reacts very slowly with water but faster with warm water
C) Iron will not react with water in the absence of air
D) Sodium reacts with water
E) Copper reacts with steam
Show Answer
The correct answer is E .
A) Drug making
B) Cement making
C) Paint making
D) Perfume making
Show Answer
The correct answer is A .
A) chlorination
B) passage over activated charcoal
C) the use of an ion-exchange resin
D) aeration
Show Answer
The correct answer is C .
What happens to the position of equilibrium, if a reversible exothermic reaction is subjected to a decrease in temperature?
Options:A) The position of equilibrium shifts to the left
B) The position of equilibrium shifts to the right.
C) The position of equilibrium remains unchanged
D) The reaction stops
Show Answer
The correct answer is B .