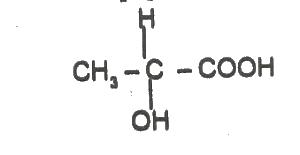
The compound above exhibits
Options:A) geometric isomerism
B) optical isomerism
C) structural isomerism
D) positional isomerism
Show Answer
The correct answer is B .
Hydrogen bond is a sort of
Options:A) van der waals force
B) dative bond
C) ionic bond
D) a covalent bond
Show Answer
The correct answer is D .
The dehydration of CH
A) HC≡CCH
B) CH
C) CH
D) CH
Show Answer
The correct answer is D .
A) a decrease in the free energy of the solution
B) a decrease in the entropy of the solution
C) a lowering of the temperature of te solution
D) an increase in the entropy of the solution
Show Answer
The correct answer is D .

The functional groups present in the compound above are
Options:A) alkene and halo-group
B) hydroxyl and chloro-group
C) alkene and chloro-group
D) hydroxyl and halo-group
Show Answer
The correct answer is B .

Choose the correct answer from the options above.
When Fehling's solution is added to two isomeric carbonyl compounds X and Y with the molecular formula C 5H 10O, compound X gives a red precipitate while Y does not react. It can be inferred that X is
Options:A) A
B) B
C) C
D) D
Show Answer
The correct answer is B .
A)
B) X-rays
C)
D)
Show Answer
The correct answer is C .
A) CuO (S).
B) MgCL2(S).
C) CaCL2(S).
D) NaOH(S).
Show Answer
The correct answer is A .
SO
In the reaction above, the most suitable catalyst is?
Options:A) chromium(vi)oxide
B) iron(iii)oxide
C) copper(i)oxide
D) vanadium(v)oxide
Show Answer
The correct answer is D .
A) Propanel
B) Ethanol
C) Methanoic acid
D) Glucose
Show Answer
The correct answer is B .