A) Copper
B) Iron
C) Lead
D) Potassium
E) Zinc
Show Answer
The correct answer is D .
What is the state of matter in which particles are widely spaced and move freely with high kinetic energy?
Options:A) Liquid
B) Solid
C) Plasma
D) Gas
Show Answer
The correct answer is D .
A) 3
B)
C) 1
D) 2
Show Answer
The correct answer is C .
A) 4.0 χ 10-5
B) 0.4 χ 10-5
C) 4.0 χ 10-3
D) 0.4 χ 10-3
Show Answer
The correct answer is A .
A) CH3COOH
B) CH3COOCH3
C) CH3COOC2H5
D) C2H5COOCH3
Show Answer
The correct answer is A .
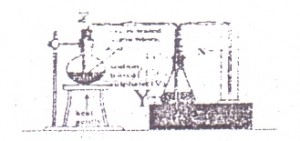
In the diagram above. X is
Options:A) SO3
B) SO2
C) S
D) H2S
Show Answer
The correct answer is B .
Electrons enter into orbitals in order of increasing energy as exemplified by?
Options:A) 1S
B) 1S
C) 1S
D) 1S
Show Answer
The correct answer is B .
A) Chlorine gas and hydrogen
B) Oxygen and oxochlorate (I) acid
C) Chlorine gas and oxochlorate (I) acid
D) Hydrochloric acid and oxygen
Show Answer
The correct answer is D .
A) Y
B) Z
C) Y - Z
D) Z - Y
Show Answer
The correct answer is B .
A) oxygen
B) moisture
C) carbondioxide
D) oxygen and moisture
E) oxygen, moisture and carbondioxide
Show Answer
The correct answer is D .