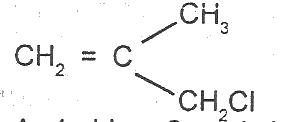
What is the IUPAC name for the compound?
Options:A) 1-chloro-2-methylprop-2,3-ene
B) 1-chloro-2-methylprop-2-ene
C) 3-chloro-2-methylprop-1-ene
D) 3-chloro-2methylprop-1,2-ene
Show Answer
The correct answer is C .
A) dative bond
B) metallic bond
C) hydrogen bond
D) van der Waal's forces
Show Answer
The correct answer is D .
A) 135.0cm
B) 180.0cm
C) 45.0cm
D) 190.0cm
E) 90.0cm
Show Answer
The correct answer is E .
Electrons enter into orbitals in order of increasing energy as exemplified by?
Options:A) 1S
B) 1S
C) 1S
D) 1S
Show Answer
The correct answer is B .
An electron can be added to a halogen atom to form a halide ion with?
Options:A) 8 valence electrons
B) 7 valence electrons
C) 2 valence electrons
D) 3 valence electrons
Show Answer
The correct answer is A .
A) electrons
B) Holes and electron
C) Ions
D) Charges
Show Answer
The correct answer is C .
Soaps clean surfaces on the principlebased on?
Options:A) viscosity
B) floatation
C) elasticity
D) surface tension
Show Answer
The correct answer is D .
Zn + H2 SO4 → ZnSO4 + H2
In the above reaction how much Zinc will be left of 1.0 M of H2SO?
[Zn =65, s = 32, O = 16, H = 1]
Options:A) 1.35g
B) 1.00g
C) 0.70g
D) 0.65g
E) 0.00g
Show Answer
The correct answer is A .
A basic postulate of the kinetic theory of gases is that the molecules of a gas move in straight lines between collisions. This implies that
Options:A) collisions are perfectly elastic
B) forces of repulsion exist
C) forces of repulsion and attraction are in equilibrium
D) collisions are inelastic
Show Answer
The correct answer is B .
The heat required to raise the temperature of the body by 1k is called?
Options:A) Specific heat
B) Thermal capacity
C) Water equivalent
D) None of the above
Show Answer
The correct answer is B .