A) HNO 3 +NaOH
B) H 2SO 4 + KOH
C) HC +Mg (OH) 2
D) HNO 3 + KOH
Show Answer
Which of the following reactions would be expected to have the highest entropy change?
Options:A) Liquid → Gas
B) Solid → Liquid
C) Gas → Liquid
D) Gas → Solid
Show Answer
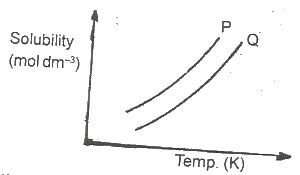
In the diagram above, the mixture of the solids P and Q can be separated by
Options:A) distillation
B) fractional distillation
C) crystallization
D) fractional crystallization
Show Answer
A) ethanol
B) glycerol
C) methanol
D) propanol
E) glycol
Show Answer
A radioactive nucleus has a half-life of 20 years, starting with 100,000 particles, how many particles will be left exactly at the end of 40 years
Options:A) 75,000 particles
B) 35,000 particles
C) 25,000 particles
D) 50,000 particles
Show Answer
A) volume of water reduces
B) volume of chemical waste increase
C) level of oxides of nitrogen increase
D) level of oxygen reduces
Show Answer
CO(g) + H2 O(g) → CO2(g) + H2(g)
Calculate the standard heat change of the reaction above, if the standard enthalpies of formation of CO2(g), H2O(g) and CO(g) and CO(g) in KJ mol -1 are - 394 -242 and - 110 respectively.
Options:A) + 262 KJ mol -1
B) - 262 KJ mol -1
C) + 42 KJ mol -1
D) - 42 KJ mol -1
Show Answer
Which of the following is a primary constituent of crude oil?
Options:A) Pentane
B) Ethanol
C) Heptane
D) Methane
Show Answer
A) (CH3)2CH - CH(CH3)CH2CH3
B) CH3CH2(CH3)CHCH2CH2CH3
C) CH3CH2(CH3)CHCH2CH2CH3
D) (CH3)3C - CH2CH2CH3
Show Answer
A) nitrogen
B) aluminium
C) copper
D) sulphur
Show Answer