A) a covalent bond
B) an ionic bond
C) a dative covalent bond
D) a hydrogen bond
Show Answer
The correct answer is D .
What is the IUPAC name for the compound CCl
A) Tetrahydrochloride
B) Tetrachloromethane
C) Carbon tetrachloride
D) Chloroform
Show Answer
The correct answer is B .
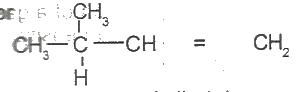
The IUPAC name for
Options:A) 2-methylbut-3-ene
B) 2-methylbut-4-ene
C) 3-methylbut-2-ene
D) 3-methylbut-1-ene
E) 3-methylpent-1-ene
Show Answer
The correct answer is D .
A) C1H2n-2
B) C1H2n-1CHO
C) C1H2n-2OH
D) C2H2n+1OH
Show Answer
The correct answer is D .
A) 49.19 cm 3
B) 48.87 cm 3
C) 48.55 cm 3
D) 48.23 cm 3
Show Answer
The correct answer is D .
A) volume
B) temperature
C) vapour
D) pressure
E) weight
Show Answer
The correct answer is A .
Which of the following types of bonding does not involve the formation of new substances?
Options:A) Metallic
B) Covalent
C) Co-ordinate
D) Electrovalent
Show Answer
The correct answer is A .
Chromatography is used to separate components of mixture which differ in their rates of ?
Options:A) diffusion
B) migration
C) reaction
D) sedimentation
Show Answer
The correct answer is B .
Which process(es) is/are involved in the turning of starch iodide paper blue-black by chlorine gas?
Options:A) chlorine attacks the starch to give the blue-black color
B) chlorine attacks the iodide ion to give the blue-black color
C) chlorine oxidizes the iodide ion to produce iodine which attacks the starch to give the blue-black color
D) iodine attacks the starch to give the blue-black color
Show Answer
The correct answer is C .
A) a white precipitate on addition of AgNO3 and barium chloride solutions
B) a white precipiate when acidified with HCI, and then AgNO3 solution added
C) a white precipitate when acidified with dilute HNO3 and then AgNO3 solution added
D) a white precipitate when acidified with dilute H2SO4 and then barium nitrate solution added
E) a pungent smell of chlorine gas when dilute HCI and MnO2 are added
Show Answer
The correct answer is C .