A) green
B) blue
C) brick-red
D) yellow
E) lilac
Show Answer
Potassium trioxochlorate (V)has a solubility of 1.5 mol dm -3 at 45 oC.On cooling this solution to a temperature of 20 oC, the solubility was found to be 0.5 mol dm -3. What mass of KCIO 3 was crystalized out?
[K = 39, Cl = 35.5 O =16].
Options:A) 1.00g
B) 10.00g
C) 12.25g
D) 122.50g
Show Answer
Chlorine gas is commonly used in the production of which of the following industrial compounds?
Options:A) Chlorofluorocarbons (CFCs)
B) Methane
C) Ammonia
D) Ethanol
Show Answer
A) CH3 -CH2 -CHO2 -COOH
B) CH3 -C = CH
C) CH3 -CH2 -CH2 -CH3
D) CH2 -CH2 -CH3
Show Answer
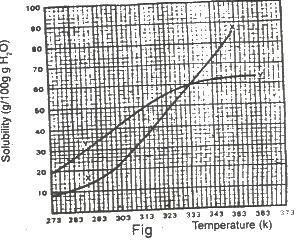
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is
Options:A) 0.2 moles
B) 0.7 moles
C) 1.5 moles
D) 2.0 moles
E) 3.0 moles
Show Answer
Which of the following metals is the most essential in the regulation of blood volume, blood pressure and osmotic equilibrium?
Options:A) Zinc
B) Manganese
C) Sodium
D) Iron
Show Answer
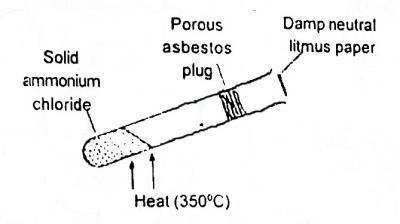
In the shown experiment (Fig. 1) the litmus paper will initially
Options:A) be bleached
B) turn green
C) turn red
D) turn blue
Show Answer
An organic compound has an empirical formula CH2O and vapour density of 45.
What is the molecular formula?
[C = 12, H = 1, O = 16]
Options:A) C3H7OH
B) C2H5OH
C) C3H6O3
D) C2H4O2
Show Answer
The pollutant usually presents in a city which generates its electricity from coal?
Options:A) fog
B) carbon(ii)oxide
C) smog
D) sulphur(iv)oxide
Show Answer
A) Ca(NO3)2
B) Cu(NO3)2
C) Mg(NO3)2
D) Al(NO3)3
Show Answer