A) XY
B) YX
C) X2Y3
D) Y2X3
Show Answer
The correct answer is D .
A) CH2O
B) C3H6O3
C) C6H6O3
D) C6H12O6
Show Answer
The correct answer is D .
A) Zin heated to redness reacts with steam to give oxygen and zinc oxide
B) Zinc heated to redness reacts with steam to give oxygen and hydrogen
C) Zinc does not react with hot or cold water
D) Zinc reacts with hot water to form zinc oxide and hydrogen
E) Zinc reacts very easily with cold water to give zinc oxide and hydrogen
Show Answer
The correct answer is D .
Which group does calcium belong to in the periodic table?
Options:A) Alkaline earth metals
B) Halogens
C) Alkali metals
D) Noble gases
Show Answer
The correct answer is A .
The contact process is used for the industrial production of
Options:A) sulfuric acid (H2SO4)
B) Hydrochloric acid (HCl)
C) Sodium hydroxide (NaOH)
D) Calcium oxide (CaO)
Show Answer
The correct answer is A .

In the graph above, the activation energy of the catalyzed reaction is
Options:A) 100KJ
B) 300KJ
C) 250KJ
D) 200KJ
Show Answer
The correct answer is A .
A) pig iron
B) wrough iron
C) cast iron
D) iron pyrite
Show Answer
The correct answer is B .
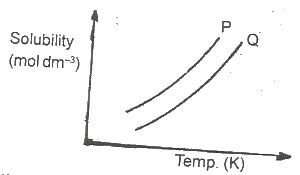
In the diagram above, the mixture of the solids P and Q can be separated by
Options:A) distillation
B) fractional distillation
C) crystallization
D) fractional crystallization
Show Answer
The correct answer is C .
Na2S2O3(aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l)
Which of the following would introduce the greatest increase in the rate of the chemical reaction above?
Options:A) An increase in temperature and decrease in the concentration of the reactant
B) A decrease in the volume and increase in the pressure of the reactant
C) A decrease in the temperature and an incease in the concentration of the reactant
D) An increase in temperature and an increase in the concentration of the reactant
Show Answer
The correct answer is D .
A) insoluble sodium compounds which from soluble solutions of calcium and magesium
B) solube soudium compounds which from soluble solutions of calcium and magnesium ions
C) solube sodium compounds which from insoluble precipitates of calcium and magnesium ions
D) insoluble precipitates of calcium and magnesium ions
Show Answer
The correct answer is C .