A) NaHSO4
B) Na2SO3
C) NaHSO3
D) Na2SO4
Show Answer
The correct answer is B .
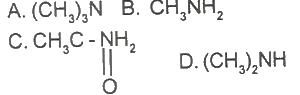
Choose the correct answer in the option above.
Which of the following is a primary amine?
Options:A) A
B) B
C) C
D) D
Show Answer
The correct answer is B .
A) -4
B) -2
C) 4
D) -8
Show Answer
The correct answer is B .
A) They have different number of carbon atoms
B) They are members of same homologous series
C) They have the same boiling points
D) They have the same chemical properties
Show Answer
The correct answer is D .
A) calcium hydroxide or magnesium hydroxide
B) calcium trioxocarbonate (IV) or calcium tetraoxosulphate (VI)
C) sodium hydroxide or magnesium ydroxide
D) calcium chloride or sodium chloride salts
Show Answer
The correct answer is B .
A) iso-octane
B) n-heptane
C) iso-heptane
D) n-octane
Show Answer
The correct answer is B .
The molar ratio of carbon to hydrogen of a volatile liquid compound is 1: 2. 0. 12 g of the liquid on evaporation at s.t.p. gave 32 cm3 of vapour. The molecular formula of the liquid is
[G.M.V =22.4 dm 3, C = 12, H = 1]
Options:A) C 3 H 6
B) C 4H 8
C) C 5C 10
D) C 6H 12
Show Answer
The correct answer is D .
Which of the following salts when dissolved in water will NOT give the corresponding pH value?
Options:A) NaHSO4, pH < 5
B) Na2CO3, pH > 8
C) Na2Cl,pH = 7
D) NaHCO, pH < 6
Show Answer
The correct answer is D .
A) O 2
B) SO 2
C) HCI
D) H 2
Show Answer
The correct answer is C .
A) magnesium trioxosilicate (IV) and rust
B) magnesium trioxosilicate (IV) and calcium tetraoxosulphate (VI)
C) clay and magnesium troxosulphate (IV)
D) clay and rust
Show Answer
The correct answer is A .