A) zinc has a more positive oxidation potential than iron
B) zinc has less positive oxidation potential than iron
C) bith have the same oxidation potential
D) zinc is harder than iron
Show Answer
A) Sp2
B) Sp2d
C) Sp3
D) Sp
Show Answer
A) the atomic number
B) the avogadro number
C) the gas constant
D) the number of neutrons
E) the number of electrons
Show Answer
When 10g of sodium hydroxide is dissolved in 1000cm3 of water, the sodium formed is approximately
[Na = 23, H = 1, O = 16]
Options:A) 0.01 mol dm-3
B) 0.10 mol dm-3
C) 0.25 mol dm-3
D) 0.50 mol dm-3
Show Answer
A given amount of gas occupies 10.0dm5 at 4atm and 273°C. The number of moles of the gas present is [Molar volume of gas at s.t.p = 22.4dm
A) 0.89 mol
B) 1.90 mol
C) 3.80 mol
D) 5.70 mol
Show Answer
What technique is suitable for separating a binary solution of potassium chloride and potassium trioxochlorate (V)?
Options:A) Fractional crystallization
B) Fractional distillation
C) Filtration
D) Evaporation
Show Answer
| GAS | CO | N | O |
| % BY VOLUME | 4 | 72 | 24 |
The above table shows the compositions of the atmosphere of planet X. Which of these gases are present inhigherpercentages on earth?
Options:A) CO
B) N
C) CO
D) O
Show Answer
The reactions below represent neutralization reaction, in which of them is the value of H highest?
Options:A) CH
B) NH
C) NaOH + HCL → NaCL + H
D) CH
Show Answer
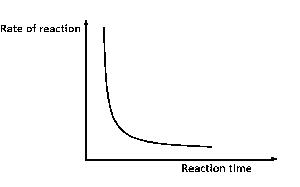
The diagram above best illustrates the effect of decrease in
Options:A) concentration
B) temperature
C) surface area
D) pressure
Show Answer
A) Ether
B) Methane
C) Butane
D) Ethene
E) Hydrogen
Show Answer