A) glucose and starch
B) maltose and starch
C) sucrose and fructose
D) maltose and starch
Show Answer
The following compounds are given with their corresponding boiling points.
Compound Boiling Point
i.................................14°C
ii................................24°C
iii................................0°C
iv................................120°C
V.................................-2°C
From the above information, indicate which of the following is correct?
Options:A) All the compounds are gaseous at 20 °C
B) One of the compounds is gaseous at 20 °C
C) Two of the compounds are gaseous at 20 °C
D) Three of the compounds are gaseous at 20 °C
E) None of the compounds is gaseous at 20 °C
Show Answer
A) 32
B) 20
C) 14
D) 12
Show Answer
Which of the following metals is the most essential in the regulation of blood volume, blood pressure and osmotic equilibrium?
Options:A) Zinc
B) Manganese
C) Sodium
D) Iron
Show Answer
A) O2
B) NO
C) SO2
D) NH3
E) CO2
Show Answer
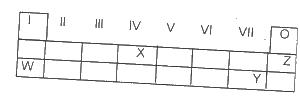
Use the table above to answer this question .The least reactive element is
Options:A) W
B) X
C) Y
D) Z
Show Answer
The mass number of a nucleus is?
Options:A) Always less than its atomic number
B) The sum of the number of protons and neutrons present in the nucleus
C) Always more than the atomic weight
D) A fraction
Show Answer
A) -4
B) -2
C) +4
D) -8
Show Answer
A) 28
B) 32
C) 44
D) 71
Show Answer
A) Silicon
B) Sulphur and phosphorus
C) Carbon
D) Chromium and nickel
Show Answer