A) Fe
B) Cu
C) Cn
D) Sn
Show Answer
The correct answer is A .
A) make baking powder
B) make fruit juices
C) remove rust
D) dry substance
Show Answer
The correct answer is A .
A) Octet configuration
B) Cyclic shape
C) Hexagonal shape
D) Obtuse configuration
Show Answer
The correct answer is A .
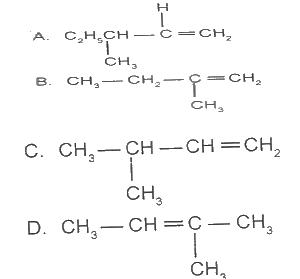
2-methylbut-2-ene has the structure
Options:A) A
B) B
C) C
D) D
Show Answer
The correct answer is D .
A) CH2O
B) C3H6O3
C) C6H6O3
D) C6H12O6
Show Answer
The correct answer is D .
Duralumin consists of aluminum, copper?
Options:A) Zinc and Gold
B) Lead and Manganese
C) Nickel and Silver
D) Manganese and Magnesium
Show Answer
The correct answer is D .
The solubility of the solids that dissolves in a given solvent with the liberation of heat will
Options:A) increase with an increase in temperature
B) decrease with an increase in temperature
C) decrease with a decrease in temperature
D) not be affected by changes in temperature
Show Answer
The correct answer is A .
A) NaHSO4
B) Na2SO4
C) CH3CO2Na
D) Na2S
Show Answer
The correct answer is A .
A) a covalent bond
B) an ionic bond
C) a dative covalent bond
D) a hydrogen bond
Show Answer
The correct answer is D .
A) NH3
B) Mg(OH)2
C) Ca(OH)2
D) NaOH
Show Answer
The correct answer is A .