Which one of the following is not a mixture?
Options:A) Air
B) Mercury
C) Milk
D) Cement
Show Answer
The correct answer is B .
How many alkoxyalkanes can be obtained from the molecular formula C
A) 4
B) 2
C) 1
D) 3
Show Answer
The correct answer is D .
The part of the total energy of a system that accounts for the useful work done in a system is known as
Options:A) Enthalpy change
B) None of the above
C) Gibb's free energy
D) Entropy
Show Answer
The correct answer is C .
The solubility of the solids that dissolves in a given solvent with the liberation of heat will
Options:A) increase with an increase in temperature
B) decrease with an increase in temperature
C) decrease with a decrease in temperature
D) not be affected by changes in temperature
Show Answer
The correct answer is A .
A) 66%
B) 25%
C) 40%
D) 50%
Show Answer
The correct answer is D .
A) AlCl3
B) NH4Cl
C) Na2CO3
D) CH3COONa
Show Answer
The correct answer is A .
The nucleus of an atom consists of?
Options:A) Electrons and neutrons
B) Electrons and protons
C) Protons and neutrons
D) None of the above
Show Answer
The correct answer is C .
8g of CH
A) 3.7dm
B) 11.2dm
C) 22.4dm
D) 33dm
Show Answer
The correct answer is B .
A) precipitate aluminium hdroxide
B) lower the melting point of aluminium oxide
C) act as a raw materials
D) act as a solvent
Show Answer
The correct answer is B .
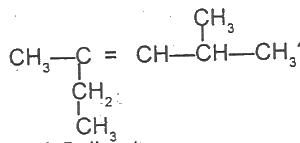
What is the IUPAC name for the hydrocarbon?
Options:A) 2-ethyl-4-methylpent-2-ene
B) 3,5-dimethylhex-3-ene
C) 2,4-dimethylhex 3-ene
D) 2-methyl-4-ethylpent-3-ene
Show Answer
The correct answer is A .