A solution X, on mixing with AgNO
A) Al
B) Hg
C) Pb
D) Ag
Show Answer
The correct answer is C .
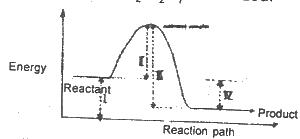
The diagram above shows the reaction path of an exothermic reaction. The heat of reaction is represented by
Options:A) l
B) ll
C) lll
D) lV
Show Answer
The correct answer is D .
A) 4.0 χ 10-5
B) 0.4 χ 10-5
C) 4.0 χ 10-3
D) 0.4 χ 10-3
Show Answer
The correct answer is A .
A) 0.5 mol dm3
B) 0.6 mol dm3
C) 0.2 mol dm3
D) 0.4 mol dm3
Show Answer
The correct answer is A .
A) Neon
B) Radon
C) Xenon
D) Argon
Show Answer
The correct answer is B .
A) sodium
B) chlorine
C) hydrogen
D) sodium hydroxide
E) oxygen
Show Answer
The correct answer is C .
A) 2.0cm3
B) 5.0cm3
C) 6.8cm3
D) 8.3cm3
Show Answer
The correct answer is A .
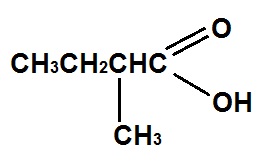
The IUPAC name for
Options:A) 2-methylbutanoic acid
B) 2-methyl-1- hydroxyketone
C) 2-methyl-1-hydroxy aldehyde
D) 2-methylpentanoic acid
Show Answer
The correct answer is A .
Which of the following is a common laboratory indicator for bases?
Options:A) Methyl orange
B) Phenolphthalein
C) Bromothymol blue
D) Litmus
Show Answer
The correct answer is B .
A) Cl-
B) NO3-
C) SO32-
D) SO42-
Show Answer
The correct answer is C .