By what amount must the temperature of 200cm
A) 150°C
B) 177°C
C) 75°C
D) 120°C
Show Answer

Which of the five atoms can be described by the following properties: Relative atomic mass is greater than 30 but less than 40, it has odd atomic number and forms a unipositive ion in solution?
Options:A) T
B) W
C) X
D) Y
E) Z
Show Answer
To what volume must 300cm
A) 450cm
B) 300cm
C) 200cm
D) 150cm
Show Answer
A) one isomer
B) two isomers
C) three isomers
D) four isomers
Show Answer
A chemical widely used as a fertilizer is?
Options:A) galena
B) bauxite
C) emerald
D) nitrochalk
Show Answer
A) 6.2
B) 6.8
C) 7.1
D) 6.9
Show Answer
CuO(s) + H2(g) ↔ Cu(s) + H2O(l)
What is the effect of increasing the pressure on the equilibrium reaction above?
Options:A) The equilibrium is shifted to the left
B) The equilibrium is shifted to the right
C) There is no effect
D) More H2(g) is produced
Show Answer
2H2S(g) + SO2(g) + H2O(l) → 3S(s) + 3H2O(l)..........(I)
3CuO(s) + 2NH3(g) → 3Cu(s) + 3H2O(l) + N2(g).........(II)
In the equations above, the oxidizing agent (I) and the reducing agent in (II) respectively are?
Options:A) H2S and NH3
B) SO2 and CuO
C) SO2 and NH3
D) H2S and CuO
Show Answer
Which of the following is a common property of non-metals?
Options:A) Exist as solids at room temperature
B) Tend to gain electrons in chemical reactions
C) High thermal conductivity
D) Readily form cations in chemical reactions
Show Answer
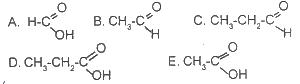
Which of the structural forms above is ethanoic acid is
Options:A) A
B) B
C) C
D) D
E) E
Show Answer