A) NaHCO3
B) NaOH
C) Na2CO3
D) NaCl
Show Answer
| Temp (°C) | 25 | 35 | 45 |
| Time (seconds) | 72 | 36 | 18 |
The times taken for iodine to be liberated in the reaction between sodium thiosulphate and hydrochloric acid at various temperature are as follows. These results suggest that
Options:A) for a 10o rise in temperature, rate of reaction is double
B) for a 10 o rise in temperature, rate of reaction is halved
C) time taken for iodine to appear does not depend on temperature
D) for a 10 o rise in temperature rate of reaction is tripled
Show Answer
An unknown organic compound X has a relative molecular mass of 180. It is a colourless crystaline solid, readily soluble in water. X contains the elements C, H and O in the atomic ratio 1: 2:1. The compound has a sweet teste and melts on heating. In the presence of yeast and in the absence of air, X is converted to compound Y and a colourless gas. Compound Y reacts with sodium metal to produce a gas Z which gives a 'pop' sound with a glowing splint. Y also reacts with ethanoic acid to give a sweet smelling compound W.
The molecular formula of X is
Options:A) C12H22O11
B) C6H12O6
C) C3H6O 3
D) C7H14O7
E) C12H6O4
Show Answer
A) increase temperature at constant pressure
B) dcrease pressure at constant temperature
C) cool down the apparatus with water
D) decreases temperature at constant pressure
Show Answer
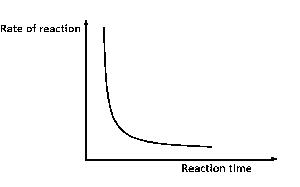
The diagram above best illustrates the effect of decrease in
Options:A) concentration
B) temperature
C) surface area
D) pressure
Show Answer
Methanoic acid mixes with water in all proportions and has about the same boiling point as water. Which of the following methods would you adopt to obtain pure water from a mixture of Sand, water and methanoic acid?
Options:A) Fractional distillation
B) Filtration followed by distillation
C) Neutralization with sodium hydroxide followed by distillation
D) Neutralization with sodium hydroxide followed by filtration
Show Answer
What is the PH of 0.00 1 moldm
A) 14
B) 13
C) 12
D) 11
Show Answer
A) Fe
B) Al
C) Zn
D) Pb
E) none of the options is correct
Show Answer
A) Methyl methanoate and ethene
B) Metanoic acid and ethyne
C) Ethyl methanoate and ethyne
D) Methyl methanoate and ethyne
E) Ethanoic acid and ethene
Show Answer
A) butane gas flame
B) acetylene flame
C) kerosene flame
D) oxy-acetylene flame
E) oxygen flame
Show Answer