What is the sum of the oxidation numbers in a neutral compound?
Options:A) +2
B) -1
C) 0
D) +1
Show Answer
The correct answer is C .
A) 5 g of lumps of CacO3 at 25°C
B) 5 g of powered CacO3 at 25°
C) 5 g of lumps of CaCO3 at 50°C
D) 5 g of powered CaCO3 AT 50°C
Show Answer
The correct answer is D .
A) 60 cm3
B) 10 cm3
C) 40 cm3
D) 30 cm3
E) 70 cm3
Show Answer
The correct answer is A .
A) Low melting point
B) Weak electropositive character
C) High boiling point
D) White lustrous appearance
Show Answer
The correct answer is A .
A) Kinetic energy of particles increases from solid to gas
B) Random motion of particles increases from gas to solid
C) Orderliness of particles increases from gas to liquid
D) Random motion of particles increases from liquid to gas
Show Answer
The correct answer is B .
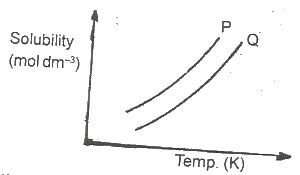
In the diagram above, the mixture of the solids P and Q can be separated by
Options:A) distillation
B) fractional distillation
C) crystallization
D) fractional crystallization
Show Answer
The correct answer is C .
Zn + 2HCL ZnCl
What happens to zinc in the above reaction?
Options:A) oxidized
B) a reactant
C) reduced
D) a metal
Show Answer
The correct answer is A .
A gaseous metallic chloride MCl consists of 20.22% of M by mass. The formula of the chloride is?
[ M = 27, Cl = 35.5]
Options:A) MCl
B) MCl
C) MCl
D) M
Show Answer
The correct answer is C .
A) an electron transferred from NH3 + H+
B) an electron pair donated by NH3
C) an electron pair equally shared
D) an electron pair donated by H+
E) an electron tarnsferred from H+ to NH3
Show Answer
The correct answer is B .
A) helps to spread the oil over a larger surface area
B) react with the oil to form an odourless compound
C) makes the oil evaporate easily by dissolving it
D) helps to dilute the oil and reduce its colour
Show Answer
The correct answer is C .