A) Aluminium oxide
B) Sodium hydrogentrioxosulphate (V)
C) Sodium trioxocarbonate (V)
D) Zinc chloride
Show Answer
The correct answer is A .
The refreshing and characteristic taste of soda water and other soft drinks is as a result of the presence of
Options:A) carbon(IV)oxide
B) carbon(ll)oxide
C) soda
D) glucose
Show Answer
The correct answer is A .
A) dissolving it in water, recrystallizing it and then drying in the oven
B) passing it through a concentrated aqueous solution of sodium hydroxide
C) passing it through calcium oxide
D) passing it through concentrated sulphuric acid
E) passing it through anhydrous calcium choride
Show Answer
The correct answer is C .
A) boiling point
B) hydrogen bonding
C) ionic character
D) covalent nature
Show Answer
The correct answer is B .
A) Xenon
B) Neon
C) Helium
D) Argon
Show Answer
The correct answer is D .
A) silver
B) copper
C) iron
D) aluminium
Show Answer
The correct answer is B .
Which of the following types of alkanols undergo oxidation to produce alkanoic acids.I. Primary alkanols
II. Secondary alkanols
III. Tertiary alkanols
Options:A) I, II and III
B) I and II only
C) III only
D) I only
Show Answer
The correct answer is D .
Potassium trioxochlorate (V)has a solubility of 1.5 mol dm -3 at 45 oC.On cooling this solution to a temperature of 20 oC, the solubility was found to be 0.5 mol dm -3. What mass of KCIO 3 was crystalized out?
[K = 39, Cl = 35.5 O =16].
Options:A) 1.00g
B) 10.00g
C) 12.25g
D) 122.50g
Show Answer
The correct answer is D .
A) copper
B) aluminium
C) zinc
D) sodium
Show Answer
The correct answer is D .
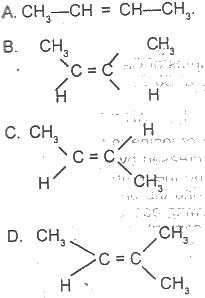
Use the graph above to answer this question. The structure of cis-2-butene is
Options:A) A
B) B
C) C
D) D
Show Answer
The correct answer is B .