An organic compound contains 69% carbon, 15.3% hydrogen and 30.7% oxygen. Calculate the the empirical formula [C=12, H = 1, O = 16]
Options:A) C
B) C
C) C
D) C
Show Answer
The correct answer is D .
A) 6.2
B) 6.8
C) 7.1
D) 6.9
Show Answer
The correct answer is D .
The heat of reaction can be determined experimentally using a device called a
Options:A) Calorimeter
B) Barometer
C) Spectrometer
D) Thermometer
Show Answer
The correct answer is A .
A) constant composition
B) conservation of mass
C) reciprocal proportions
D) multiple proportions
Show Answer
The correct answer is D .
A) Concentrated tetraoxosulphate (VI) acid
B) Anhydrous calcium chloride
C) Calcium oxide
D) Sodium hydroxide
Show Answer
The correct answer is A .
A) solid particles dispersed in liquid
B) solid or liquid particles dispersed in gas
C) gas or liquid particles dispersed inliquid
D) liquid particles dispersed inliquid
Show Answer
The correct answer is B .

In the diagram above, the gas produced is
Options:A) NO
B) NO2
C) N2O
D) N2O4
Show Answer
The correct answer is A .
A) NaHSO4
B) Na2SO4
C) CH3CO2Na
D) Na2S
Show Answer
The correct answer is A .
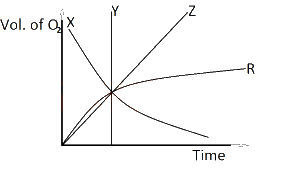
In the diagram above, which of the curves represents the evolution of oxygen with time in the equation 2KClO3(s) → 2KCl(s) + 3O2(g)?
Options:A) X
B) Y
C) Z
D) R
Show Answer
The correct answer is C .
A) basicity
B) acid strength
C) pH
D) concentration
Show Answer
The correct answer is B .