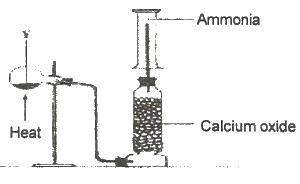
In the diagram above, Y is a mixture of
Options:A) calcium hydroxide and ammonium chloride
B) calcuim hydroxide and sodium chloride
C) sodium chloride and ammonuim trioxonitrate (V)
D) sodium dioxonitrate (lll) and ammouium chloride
Show Answer
The correct answer is A .
An isotope has an atomic number of 15 and a mass number of 31. The number of protons it contains is
Options:A) 16
B) 15
C) 46
D) 31
Show Answer
The correct answer is B .
A) calcium carbonate is formed which on reaction dissolves
B) calcium hydrogen carbonate is precipitated and then dissolves
C) calcium carbonate is formed which on reaction with further carbondioxide forms solube calcium hydrogen carbonate
D) concentration of the solution has occurred with the deposition of calcium hydroxide
E) the solution has become saturated and solid carbondioxide has been deposited
Show Answer
The correct answer is C .
What is the main environmental concern associated with sulfur dioxide emissions?
Options:A) Formation of acid rain
B) Global warming potential
C) Depletion of the stratospheric ozone
D) Depletion of the ozone layer
Show Answer
The correct answer is A .
A) Neon
B) Radon
C) Xenon
D) Argon
Show Answer
The correct answer is B .
A) 54.0g
B) 27.0g
C) 13.5g
D) 108.0g
Show Answer
The correct answer is A .
A) Electrolysis of solution of its salts
B) Decomposition of its oxides
C) Displacement from solution by an alkali metal
D) Electroiysis of fused salt
Show Answer
The correct answer is D .
A) NO2
B) NH3
C) N2O
D) NO2
Show Answer
The correct answer is B .
X + Y → Z. The rate equation for the chemical reaction above is ((-Δ[x])/Δt) = K[X]2[Y].
The overall order of the reaction is
Options:A) 0.0
B) 1.0
C) 2.0
D) 3.09
Show Answer
The correct answer is D .
In the preparation of salts, the method employed will depend on the?
Options:A) composition
B) dissociating ability
C) stability to heat
D) precipitating ability
Show Answer
The correct answer is C .