
The option above shows the PH changes for the titration of a
Options:A) strong acid versus strong base
B) weak acid versus strong base
C) strong acid versus weak base
D) weak acid versus weak base
Show Answer
The correct answer is A .
A) different physical but the same chemical properties
B) different chemical but the same physical properties
C) the same chemical and physical properties
D) different atomic structure
E) the same position in the periodic table
Show Answer
The correct answer is A .
A) turpentine
B) ammonia solution
C) ethanol
D) solution of borax in water
Show Answer
The correct answer is B .
Which type of chemical combination involves the transfer of electrons from one atom to another, resulting in the formation of oppositely charged ions?
Options:A) Ionic bonding
B) Hydrogen bonding
C) Covalent bonding
D) Metallic bonding
Show Answer
The correct answer is A .
A) 25.0cm3
B) 12.5 cm3
C) 10.0 cm3
D) 5.0cm3
Show Answer
The correct answer is C .
A) both gases change the colour of the solution from yellow to colourless
B) both gases causes sulphur to be deposited
C) only hydrogen sulphide decolourizes the solution
D) only sulphur dioxide deposits sulphur
E) only hydrogen sulphide deposits sulphur
Show Answer
The correct answer is E .
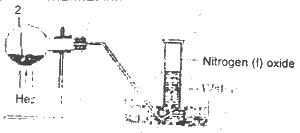
In the experiment above, Z can be
Options:A) a solution of sodium dioxonitrate (lll) and ammonium chloride
B) a solution of lead trioxnitrate (V)
C) a solution of sodium trioxonitrate (V) and ammonium chloride
D) concentrated tetraoxosulphate (VI) acid and sodium trioxonitrate (V)
Show Answer
The correct answer is C .
A) Ca(NO3)2
B) Cu(NO3)2
C) Mg(NO3)2
D) Al(NO3)3
Show Answer
The correct answer is B .
A) cause coagulation
B) neutralize acidity
C) prevent goitre and tooth decay
D) kill germs
Show Answer
The correct answer is A .
A) increase the solubility of soap
B) decrease the solubility of the soap
C) saponify the soap
D) emulsify the soap
Show Answer
The correct answer is B .