A) s orbitals
B) p orbitals
C) d orbitals
D) f orbitals
Show Answer
The correct answer is C .
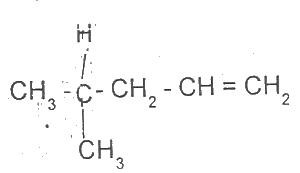
The IUPAC nomenclature for the compound above is
Options:A) 4-methylpent-1-ene
B) 3-methylpent-2-ene
C) 2-methylpent-1ene
D) 2-methylpent-4-ene
Show Answer
The correct answer is A .
What is the solubility product constant (Ksp) used for?
Options:A) To measure the total mass of a solute that can dissolve in a solvent
B) To determine the concentration of a solute in a saturated solution
C) To calculate the solubility of a solute in a given solvent
D) To compare the solubilities of different solutes in the same solvent
Show Answer
The correct answer is B .
Benzene can be converted to its derivative toluene by the addition of a methyl group. The reaction is an example of
Options:A) Nucleophilic substitution
B) Elimination reaction
C) Electrophilic substitution
D) Addition reaction
Show Answer
The correct answer is C .
The nucleusof a hydrogen atom consist of a?
Options:A) 1 proton only
B) 1 proton, 2 neutron
C) 1 neutron only
D) 1 electron only
Show Answer
The correct answer is A .
At 2.0 atm pressure, the volume of a gas is 4.0 L. If the pressure is reduced to 1.0 atm while keeping the temperature constant, what will be the new volume of the gas?
Options:A) 2.0 L
B) 6.0 L
C) 8.0 L
D) 4.0 L
Show Answer
The correct answer is C .
A) 91K
B) 182K
C) 273K
D) 819K
Show Answer
The correct answer is A .
A) solid particles dispersed in liquid
B) solid or liquid particles dispersed in gas
C) gas or liquid particles dispersed inliquid
D) liquid particles dispersed inliquid
Show Answer
The correct answer is B .
A) purer than sample 2
B) slightly denser than sample 2
C) in all respects the same as sample 2
D) colourless but sample 2 has a light brown colour
E) slightly less reactive than sample 2
Show Answer
The correct answer is B .
A) N2
B) N2O
C) CI2
D) NH3
Show Answer
The correct answer is D .